 Found 478 hits from Aventis Pharmaceuticals
Found 478 hits from Aventis Pharmaceuticals Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114539
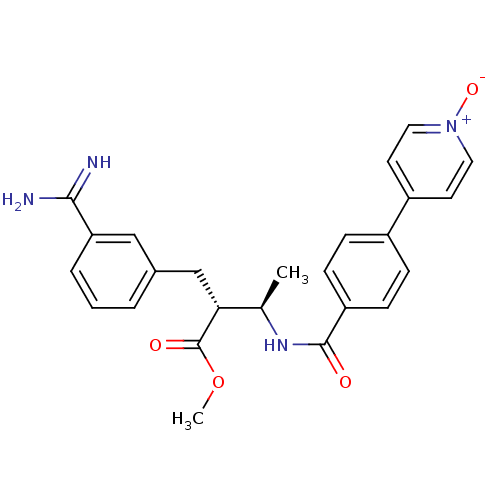
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cc[n+]([O-])cc1 Show InChI InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)15-17-4-3-5-21(14-17)23(26)27)28-24(30)20-8-6-18(7-9-20)19-10-12-29(32)13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114534
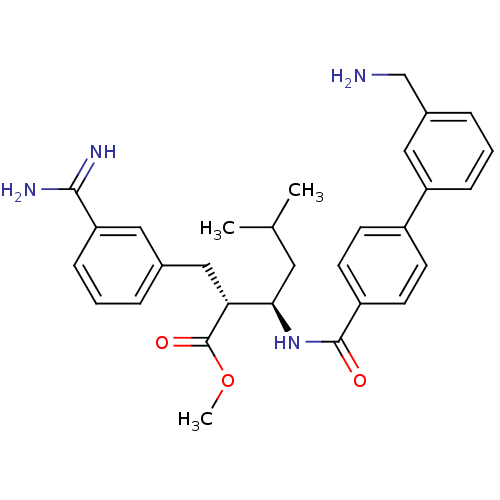
((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](CC(C)C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 Show InChI InChI=1S/C30H36N4O3/c1-19(2)14-27(26(30(36)37-3)17-20-6-4-9-25(15-20)28(32)33)34-29(35)23-12-10-22(11-13-23)24-8-5-7-21(16-24)18-31/h4-13,15-16,19,26-27H,14,17-18,31H2,1-3H3,(H3,32,33)(H,34,35)/t26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84440
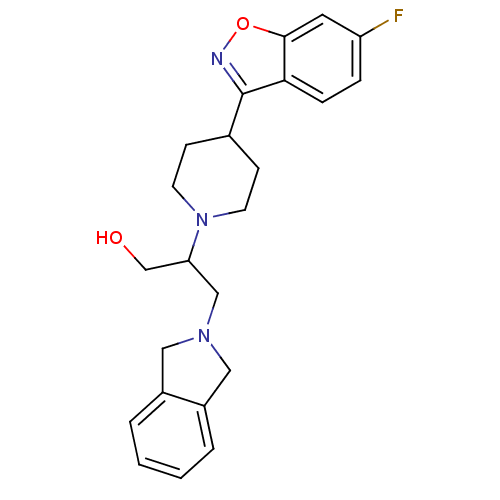
(Isoindoline, 7 | Isoindoline, 8 | Isoindoline, 9)Show SMILES OCC(CN1Cc2ccccc2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H26FN3O2/c24-19-5-6-21-22(11-19)29-25-23(21)16-7-9-27(10-8-16)20(15-28)14-26-12-17-3-1-2-4-18(17)13-26/h1-6,11,16,20,28H,7-10,12-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84445
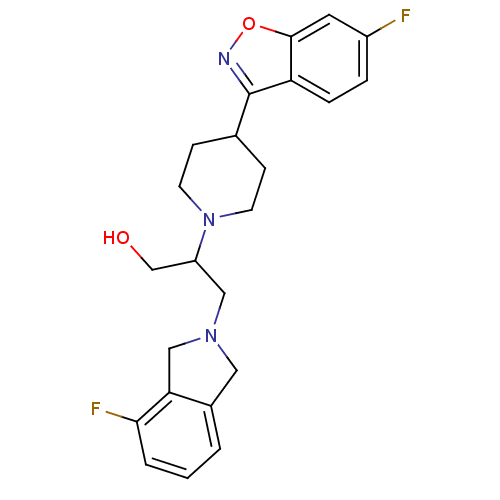
(Isoindoline, 12)Show SMILES OCC(CN1Cc2cccc(F)c2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H25F2N3O2/c24-17-4-5-19-22(10-17)30-26-23(19)15-6-8-28(9-7-15)18(14-29)12-27-11-16-2-1-3-21(25)20(16)13-27/h1-5,10,15,18,29H,6-9,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114544
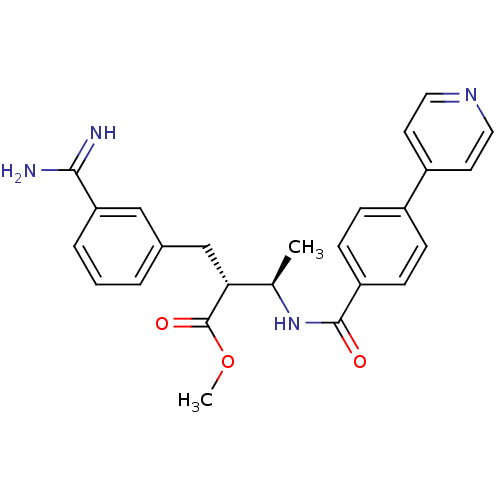
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-4-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C25H26N4O3/c1-16(22(25(31)32-2)15-17-4-3-5-21(14-17)23(26)27)29-24(30)20-8-6-18(7-9-20)19-10-12-28-13-11-19/h3-14,16,22H,15H2,1-2H3,(H3,26,27)(H,29,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085393
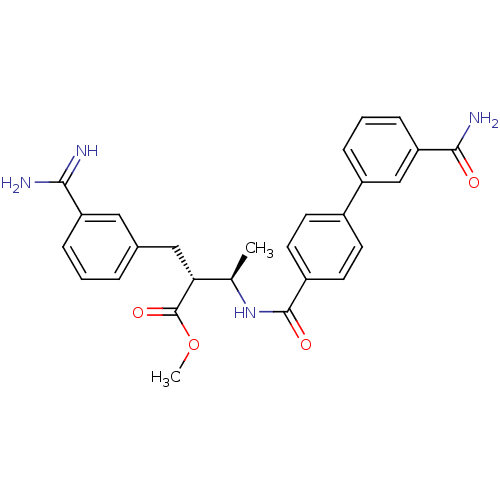
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(3'-carbamoy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(c1)C(N)=O Show InChI InChI=1S/C27H28N4O4/c1-16(23(27(34)35-2)14-17-5-3-7-21(13-17)24(28)29)31-26(33)19-11-9-18(10-12-19)20-6-4-8-22(15-20)25(30)32/h3-13,15-16,23H,14H2,1-2H3,(H3,28,29)(H2,30,32)(H,31,33)/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114543

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1[O-] Show InChI InChI=1S/C25H26N4O4/c1-16(21(25(31)33-2)15-17-6-5-7-20(14-17)23(26)27)28-24(30)19-11-9-18(10-12-19)22-8-3-4-13-29(22)32/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,28,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001786
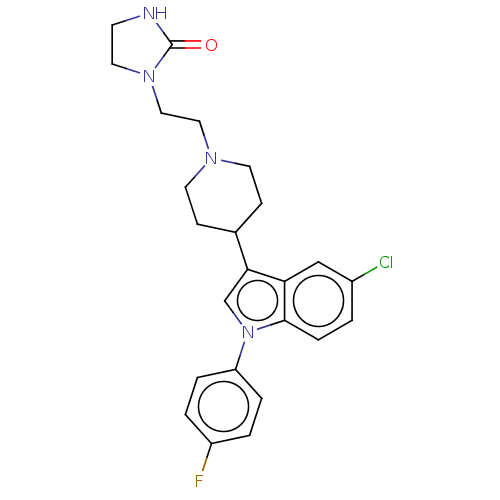
(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84451
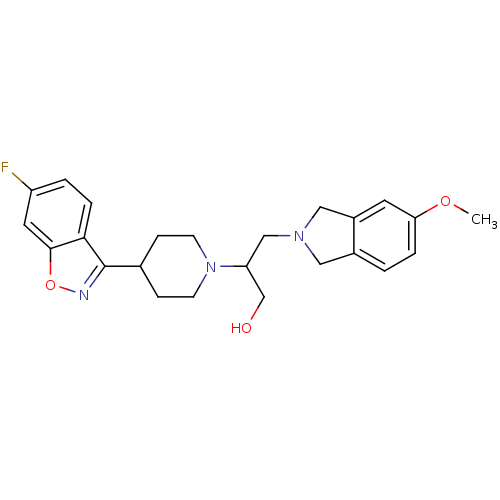
(Isoindoline, 18 | Isoindoline, 19)Show SMILES COc1ccc2CN(CC(CO)N3CCC(CC3)c3noc4cc(F)ccc34)Cc2c1 Show InChI InChI=1S/C24H28FN3O3/c1-30-21-4-2-17-12-27(13-18(17)10-21)14-20(15-29)28-8-6-16(7-9-28)24-22-5-3-19(25)11-23(22)31-26-24/h2-5,10-11,16,20,29H,6-9,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001786

(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards Serotonin 5-hydroxytryptamine 2A receptor |
J Med Chem 46: 2017-22 (2003)
Article DOI: 10.1021/jm0205651
BindingDB Entry DOI: 10.7270/Q2XD12C8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114536
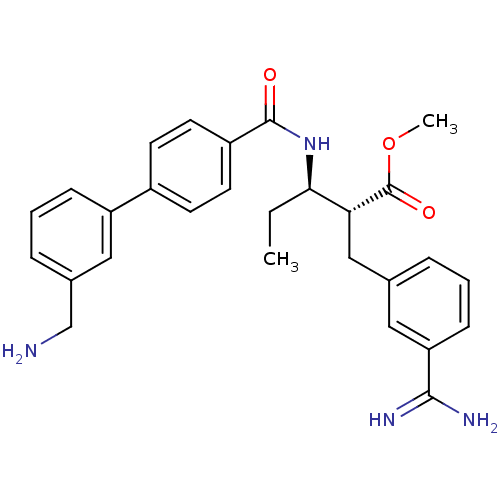
((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES CC[C@@H](NC(=O)c1ccc(cc1)-c1cccc(CN)c1)[C@@H](Cc1cccc(c1)C(N)=N)C(=O)OC Show InChI InChI=1S/C28H32N4O3/c1-3-25(24(28(34)35-2)16-18-6-4-9-23(14-18)26(30)31)32-27(33)21-12-10-20(11-13-21)22-8-5-7-19(15-22)17-29/h4-15,24-25H,3,16-17,29H2,1-2H3,(H3,30,31)(H,32,33)/t24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123781
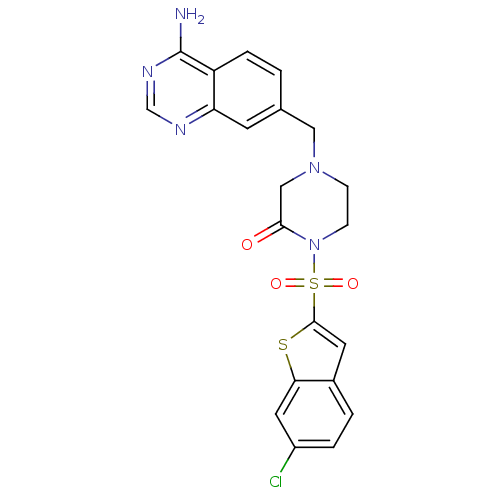
(4-(4-Amino-quinazolin-7-ylmethyl)-1-(6-chloro-benz...)Show SMILES Nc1ncnc2cc(CN3CCN(C(=O)C3)S(=O)(=O)c3cc4ccc(Cl)cc4s3)ccc12 Show InChI InChI=1S/C21H18ClN5O3S2/c22-15-3-2-14-8-20(31-18(14)9-15)32(29,30)27-6-5-26(11-19(27)28)10-13-1-4-16-17(7-13)24-12-25-21(16)23/h1-4,7-9,12H,5-6,10-11H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral dose |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114540

(3-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc[n+](C)c1 Show InChI InChI=1S/C26H28N4O3/c1-17(23(26(32)33-3)15-18-6-4-7-21(14-18)24(27)28)29-25(31)20-11-9-19(10-12-20)22-8-5-13-30(2)16-22/h4-14,16-17,23H,15H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114537
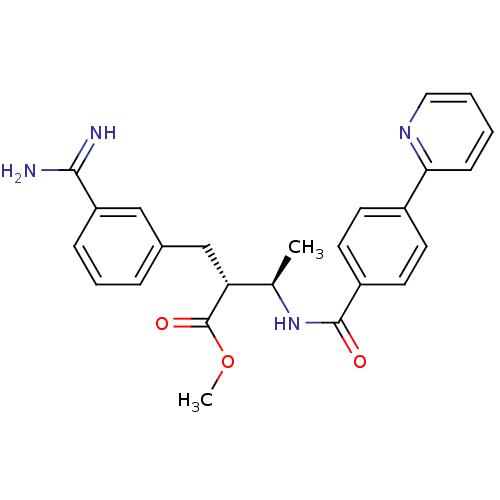
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-(4-pyridin-2-...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccccn1 Show InChI InChI=1S/C25H26N4O3/c1-16(21(25(31)32-2)15-17-6-5-7-20(14-17)23(26)27)29-24(30)19-11-9-18(10-12-19)22-8-3-4-13-28-22/h3-14,16,21H,15H2,1-2H3,(H3,26,27)(H,29,30)/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12597
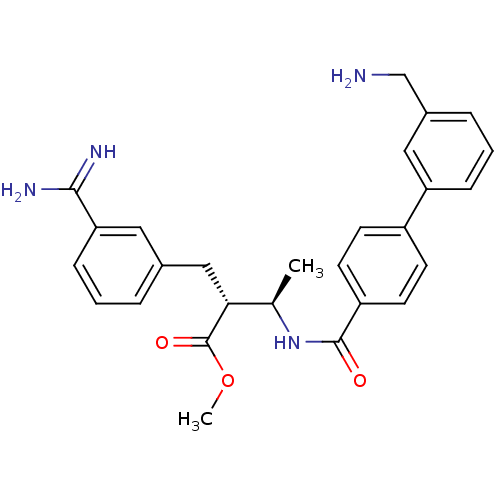
(CHEMBL48046 | RPR128515 | methyl (2R,3R)-2-{3-[ami...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 |r| Show InChI InChI=1S/C27H30N4O3/c1-17(24(27(33)34-2)15-18-5-3-8-23(13-18)25(29)30)31-26(32)21-11-9-20(10-12-21)22-7-4-6-19(14-22)16-28/h3-14,17,24H,15-16,28H2,1-2H3,(H3,29,30)(H,31,32)/t17-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84440

(Isoindoline, 7 | Isoindoline, 8 | Isoindoline, 9)Show SMILES OCC(CN1Cc2ccccc2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H26FN3O2/c24-19-5-6-21-22(11-19)29-25-23(21)16-7-9-27(10-8-16)20(15-28)14-26-12-17-3-1-2-4-18(17)13-26/h1-6,11,16,20,28H,7-10,12-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114531
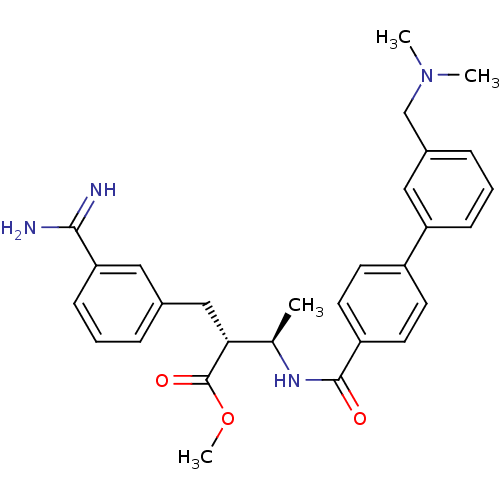
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(3'-dimethyl...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc(CN(C)C)c1 Show InChI InChI=1S/C29H34N4O3/c1-19(26(29(35)36-4)17-20-7-5-10-25(15-20)27(30)31)32-28(34)23-13-11-22(12-14-23)24-9-6-8-21(16-24)18-33(2)3/h5-16,19,26H,17-18H2,1-4H3,(H3,30,31)(H,32,34)/t19-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50111966
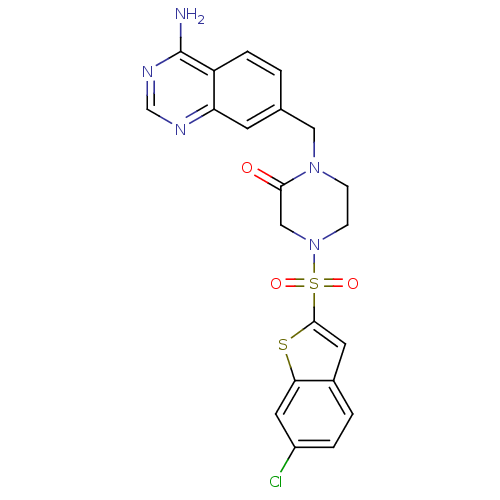
(1-(4-Amino-quinazolin-7-ylmethyl)-4-(6-chloro-benz...)Show SMILES Nc1ncnc2cc(CN3CCN(CC3=O)S(=O)(=O)c3cc4ccc(Cl)cc4s3)ccc12 Show InChI InChI=1S/C21H18ClN5O3S2/c22-15-3-2-14-8-20(31-18(14)9-15)32(29,30)27-6-5-26(19(28)11-27)10-13-1-4-16-17(7-13)24-12-25-21(16)23/h1-4,7-9,12H,5-6,10-11H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 919-22 (2002)
BindingDB Entry DOI: 10.7270/Q28C9VJT |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12594
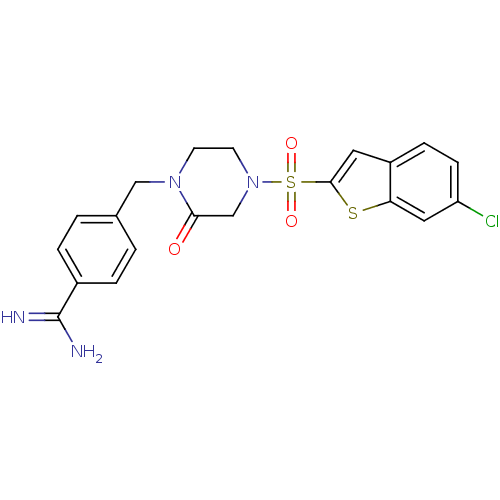
(4-({4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-2-OXO...)Show SMILES NC(=N)c1ccc(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc1 Show InChI InChI=1S/C20H19ClN4O3S2/c21-16-6-5-15-9-19(29-17(15)10-16)30(27,28)25-8-7-24(18(26)12-25)11-13-1-3-14(4-2-13)20(22)23/h1-6,9-10H,7-8,11-12H2,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 919-22 (2002)
BindingDB Entry DOI: 10.7270/Q28C9VJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12596
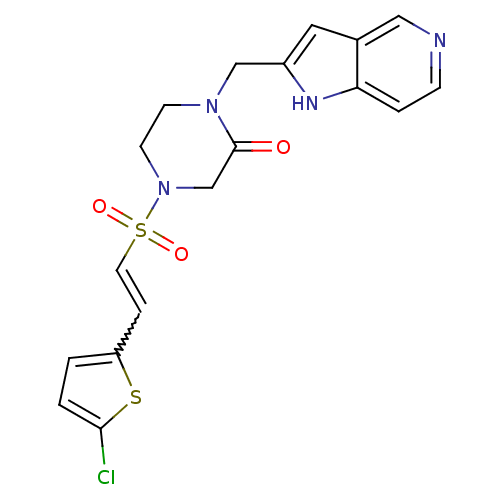
(4-{[(E)-2-(5-CHLOROTHIEN-2-YL)VINYL]SULFONYL}-1-(1...)Show SMILES Clc1ccc(C=CS(=O)(=O)N2CCN(Cc3cc4cnccc4[nH]3)C(=O)C2)s1 |w:5.4| Show InChI InChI=1S/C18H17ClN4O3S2/c19-17-2-1-15(27-17)4-8-28(25,26)23-7-6-22(18(24)12-23)11-14-9-13-10-20-5-3-16(13)21-14/h1-5,8-10,21H,6-7,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 5 mg/kg peroral dose |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84446
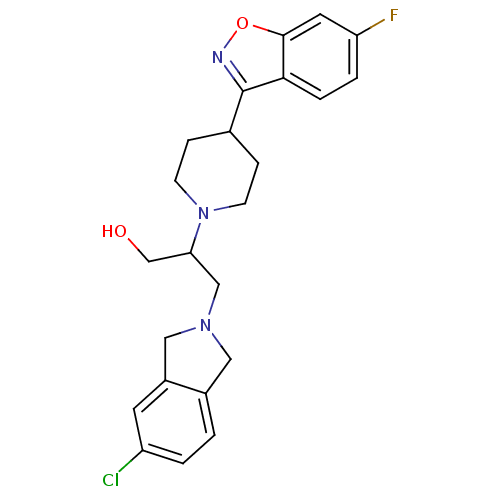
(Isoindoline, 13)Show SMILES OCC(CN1Cc2ccc(Cl)cc2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H25ClFN3O2/c24-18-2-1-16-11-27(12-17(16)9-18)13-20(14-29)28-7-5-15(6-8-28)23-21-4-3-19(25)10-22(21)30-26-23/h1-4,9-10,15,20,29H,5-8,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84443
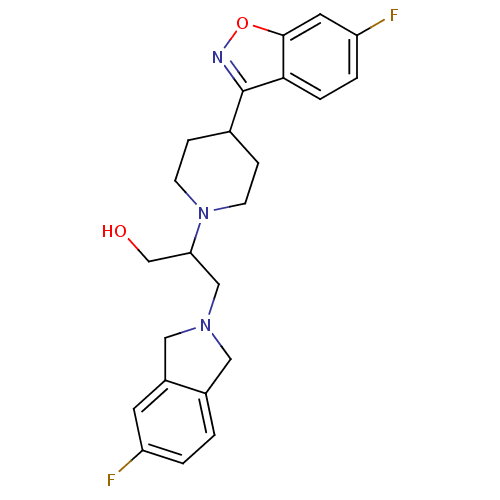
(Isoindoline, 10 | Isoindoline, 11)Show SMILES OCC(CN1Cc2ccc(F)cc2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H25F2N3O2/c24-18-2-1-16-11-27(12-17(16)9-18)13-20(14-29)28-7-5-15(6-8-28)23-21-4-3-19(25)10-22(21)30-26-23/h1-4,9-10,15,20,29H,5-8,11-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114548
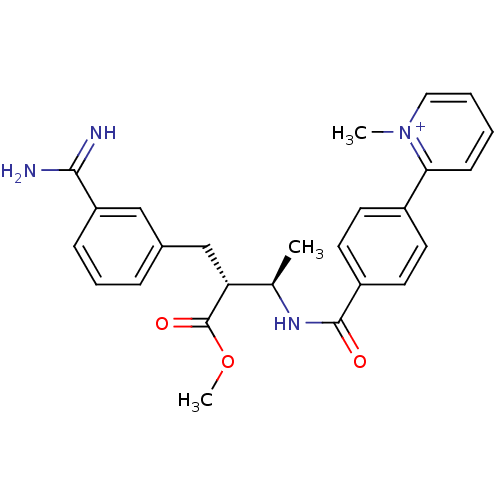
(2-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cccc[n+]1C Show InChI InChI=1S/C26H28N4O3/c1-17(22(26(32)33-3)16-18-7-6-8-21(15-18)24(27)28)29-25(31)20-12-10-19(11-13-20)23-9-4-5-14-30(23)2/h4-15,17,22H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156461
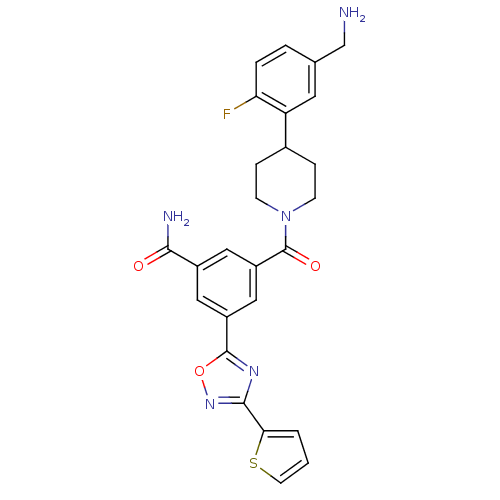
(3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H24FN5O3S/c27-21-4-3-15(14-28)10-20(21)16-5-7-32(8-6-16)26(34)19-12-17(23(29)33)11-18(13-19)25-30-24(31-35-25)22-2-1-9-36-22/h1-4,9-13,16H,5-8,14,28H2,(H2,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12594

(4-({4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-2-OXO...)Show SMILES NC(=N)c1ccc(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc1 Show InChI InChI=1S/C20H19ClN4O3S2/c21-16-6-5-15-9-19(29-17(15)10-16)30(27,28)25-8-7-24(18(26)12-25)11-13-1-3-14(4-2-13)20(22)23/h1-6,9-10H,7-8,11-12H2,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibitory activity against Coagulation factor X |
Bioorg Med Chem Lett 10: 1737-9 (2000)
BindingDB Entry DOI: 10.7270/Q23777Z2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84453
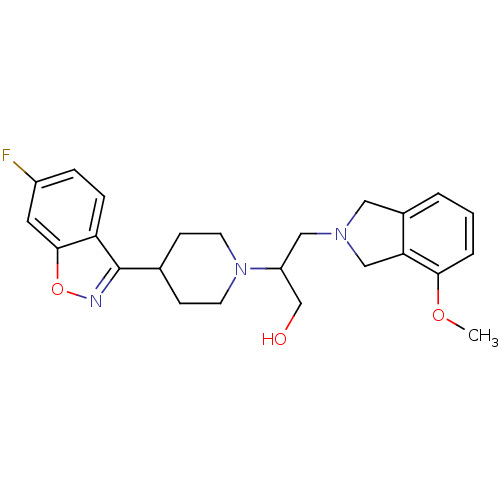
(Isoindoline, 20)Show SMILES COc1cccc2CN(CC(CO)N3CCC(CC3)c3noc4cc(F)ccc34)Cc12 Show InChI InChI=1S/C24H28FN3O3/c1-30-22-4-2-3-17-12-27(14-21(17)22)13-19(15-29)28-9-7-16(8-10-28)24-20-6-5-18(25)11-23(20)31-26-24/h2-6,11,16,19,29H,7-10,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156460
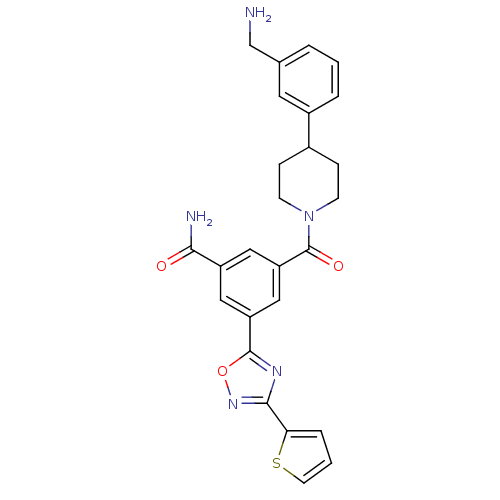
(3-[4-(3-Aminomethyl-phenyl)-piperidine-1-carbonyl]...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H25N5O3S/c27-15-16-3-1-4-18(11-16)17-6-8-31(9-7-17)26(33)21-13-19(23(28)32)12-20(14-21)25-29-24(30-34-25)22-5-2-10-35-22/h1-5,10-14,17H,6-9,15,27H2,(H2,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114547

((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1-oxy-pyr...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc[n+]([O-])c1 Show InChI InChI=1S/C25H26N4O4/c1-16(22(25(31)33-2)14-17-5-3-6-20(13-17)23(26)27)28-24(30)19-10-8-18(9-11-19)21-7-4-12-29(32)15-21/h3-13,15-16,22H,14H2,1-2H3,(H3,26,27)(H,28,30)/t16-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114528
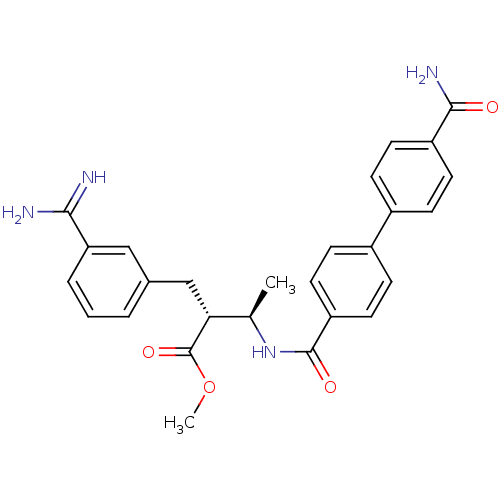
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[(4'-carbamoy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccc(cc1)C(N)=O Show InChI InChI=1S/C27H28N4O4/c1-16(23(27(34)35-2)15-17-4-3-5-22(14-17)24(28)29)31-26(33)21-12-8-19(9-13-21)18-6-10-20(11-7-18)25(30)32/h3-14,16,23H,15H2,1-2H3,(H3,28,29)(H2,30,32)(H,31,33)/t16-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114542
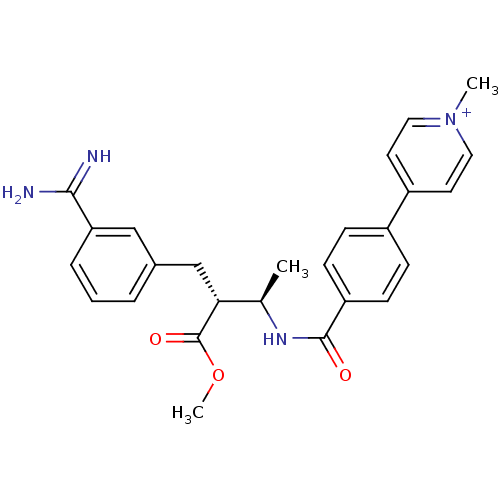
(4-{4-[(1R,2R)-3-(3-Carbamimidoyl-phenyl)-2-methoxy...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1cc[n+](C)cc1 Show InChI InChI=1S/C26H28N4O3/c1-17(23(26(32)33-3)16-18-5-4-6-22(15-18)24(27)28)29-25(31)21-9-7-19(8-10-21)20-11-13-30(2)14-12-20/h4-15,17,23H,16H2,1-3H3,(H3-,27,28,29,31)/p+1/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114538
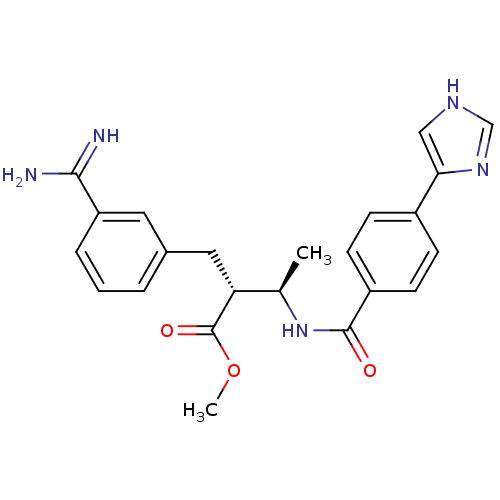
((2R,3R)-2-(3-Carbamimidoyl-benzyl)-3-[4-(1H-imidaz...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1c[nH]cn1 Show InChI InChI=1S/C23H25N5O3/c1-14(19(23(30)31-2)11-15-4-3-5-18(10-15)21(24)25)28-22(29)17-8-6-16(7-9-17)20-12-26-13-27-20/h3-10,12-14,19H,11H2,1-2H3,(H3,24,25)(H,26,27)(H,28,29)/t14-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1671-4 (2002)
BindingDB Entry DOI: 10.7270/Q2BV7FXG |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12595

(4-[(6-CHLORO-1-BENZOTHIEN-2-YL)SULFONYL]-1-{[1-(2-...)Show SMILES OCCn1c(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc2cnccc12 Show InChI InChI=1S/C22H21ClN4O4S2/c23-17-2-1-15-10-22(32-20(15)11-17)33(30,31)26-6-5-25(21(29)14-26)13-18-9-16-12-24-4-3-19(16)27(18)7-8-28/h1-4,9-12,28H,5-8,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity (in vitro) of the compound towards human Coagulation factor X was determined at 10 mg/kg peroral dose |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50111958
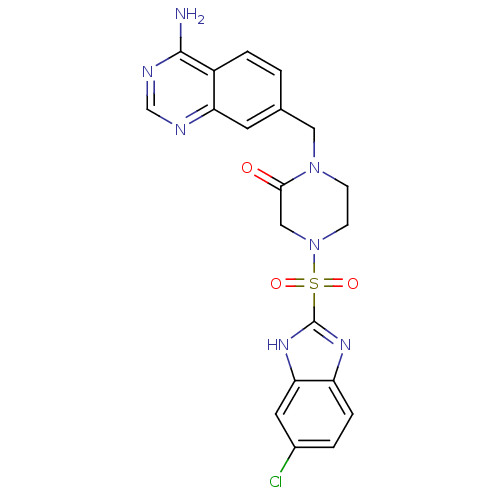
(1-(4-Amino-quinazolin-7-ylmethyl)-4-(6-chloro-1H-b...)Show SMILES Nc1ncnc2cc(CN3CCN(CC3=O)S(=O)(=O)c3nc4ccc(Cl)cc4[nH]3)ccc12 Show InChI InChI=1S/C20H18ClN7O3S/c21-13-2-4-15-17(8-13)26-20(25-15)32(30,31)28-6-5-27(18(29)10-28)9-12-1-3-14-16(7-12)23-11-24-19(14)22/h1-4,7-8,11H,5-6,9-10H2,(H,25,26)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor Xa |
Bioorg Med Chem Lett 12: 919-22 (2002)
BindingDB Entry DOI: 10.7270/Q28C9VJT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50001786

(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123766

(4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-(1-met...)Show SMILES Cn1c(CN2CCN(CC2=O)S(=O)(=O)c2cc3ccc(Cl)cc3s2)cc2cnccc12 Show InChI InChI=1S/C21H19ClN4O3S2/c1-24-17(8-15-11-23-5-4-18(15)24)12-25-6-7-26(13-20(25)27)31(28,29)21-9-14-2-3-16(22)10-19(14)30-21/h2-5,8-11H,6-7,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human Coagulation factor X |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards histamine H1 receptor |
J Med Chem 46: 2017-22 (2003)
Article DOI: 10.1021/jm0205651
BindingDB Entry DOI: 10.7270/Q2XD12C8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123788
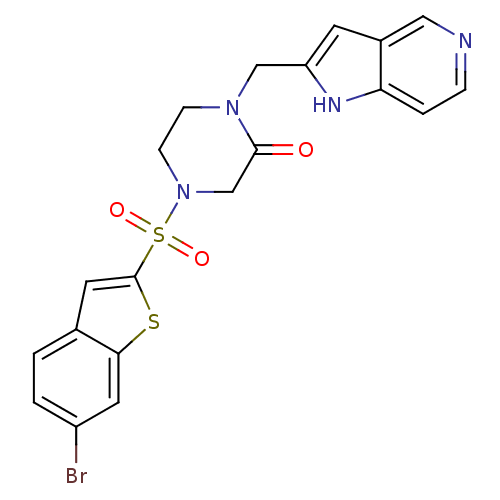
(4-(6-Bromo-benzo[b]thiophene-2-sulfonyl)-1-(1H-pyr...)Show SMILES Brc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(Cc2cc3cnccc3[nH]2)C(=O)C1 Show InChI InChI=1S/C20H17BrN4O3S2/c21-15-2-1-13-8-20(29-18(13)9-15)30(27,28)25-6-5-24(19(26)12-25)11-16-7-14-10-22-4-3-17(14)23-16/h1-4,7-10,23H,5-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human Coagulation factor X |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84459
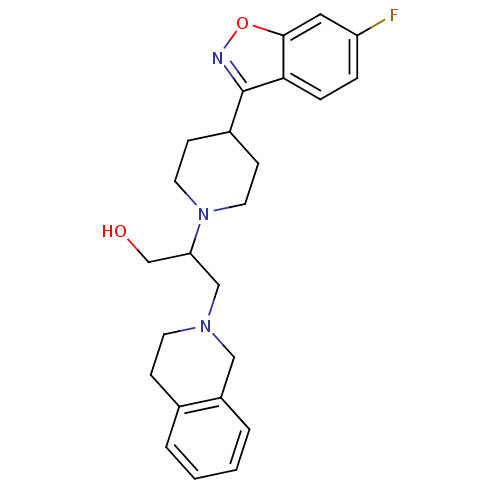
(Isoindoline, 26)Show SMILES OCC(CN1CCc2ccccc2C1)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C24H28FN3O2/c25-20-5-6-22-23(13-20)30-26-24(22)18-8-11-28(12-9-18)21(16-29)15-27-10-7-17-3-1-2-4-19(17)14-27/h1-6,13,18,21,29H,7-12,14-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84449
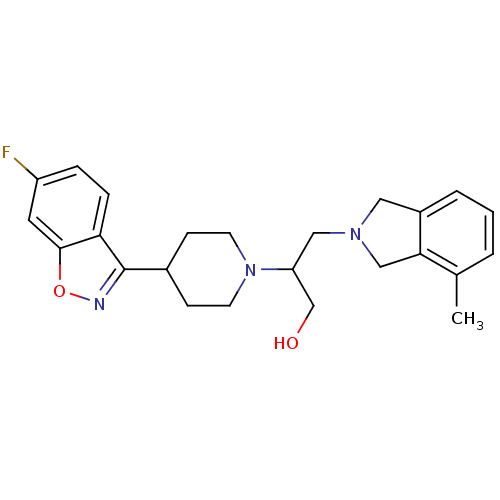
(Isoindoline, 16)Show SMILES Cc1cccc2CN(CC(CO)N3CCC(CC3)c3noc4cc(F)ccc34)Cc12 Show InChI InChI=1S/C24H28FN3O2/c1-16-3-2-4-18-12-27(14-22(16)18)13-20(15-29)28-9-7-17(8-10-28)24-21-6-5-19(25)11-23(21)30-26-24/h2-6,11,17,20,29H,7-10,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123767
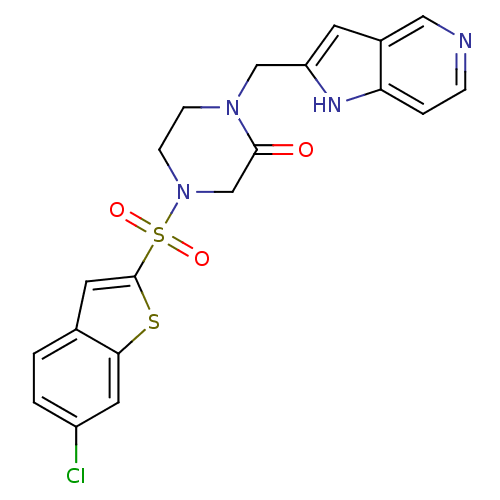
(4-(6-Chloro-benzo[b]thiophene-2-sulfonyl)-1-(1H-py...)Show SMILES Clc1ccc2cc(sc2c1)S(=O)(=O)N1CCN(Cc2cc3cnccc3[nH]2)C(=O)C1 Show InChI InChI=1S/C20H17ClN4O3S2/c21-15-2-1-13-8-20(29-18(13)9-15)30(27,28)25-6-5-24(19(26)12-25)11-16-7-14-10-22-4-3-17(14)23-16/h1-4,7-10,23H,5-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human Coagulation factor X |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50123782
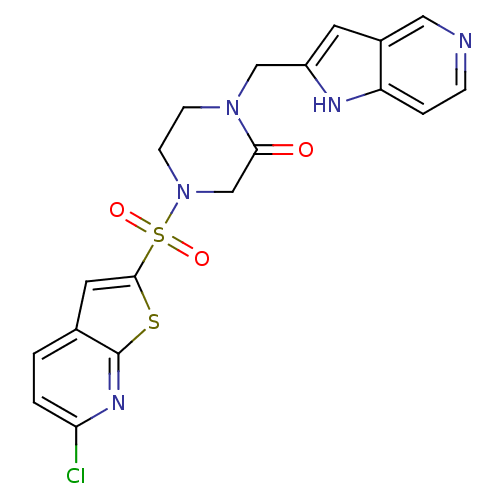
(4-(6-Chloro-thieno[2,3-b]pyridine-2-sulfonyl)-1-(1...)Show SMILES Clc1ccc2cc(sc2n1)S(=O)(=O)N1CCN(Cc2cc3cnccc3[nH]2)C(=O)C1 Show InChI InChI=1S/C19H16ClN5O3S2/c20-16-2-1-12-8-18(29-19(12)23-16)30(27,28)25-6-5-24(17(26)11-25)10-14-7-13-9-21-4-3-15(13)22-14/h1-4,7-9,22H,5-6,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro binding affinity for human Coagulation factor X |
J Med Chem 46: 681-4 (2003)
Article DOI: 10.1021/jm020384z
BindingDB Entry DOI: 10.7270/Q2KW5FDH |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50114530
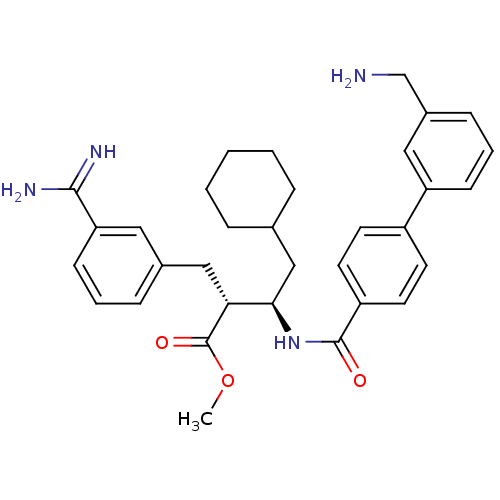
((2R,3R)-3-[(3'-Aminomethyl-biphenyl-4-carbonyl)-am...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](CC1CCCCC1)NC(=O)c1ccc(cc1)-c1cccc(CN)c1 Show InChI InChI=1S/C33H40N4O3/c1-40-33(39)29(19-23-9-5-12-28(17-23)31(35)36)30(20-22-7-3-2-4-8-22)37-32(38)26-15-13-25(14-16-26)27-11-6-10-24(18-27)21-34/h5-6,9-18,22,29-30H,2-4,7-8,19-21,34H2,1H3,(H3,35,36)(H,37,38)/t29-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |
Alpha-1B adrenergic receptor
(Rattus norvegicus (rat)) | BDBM84456
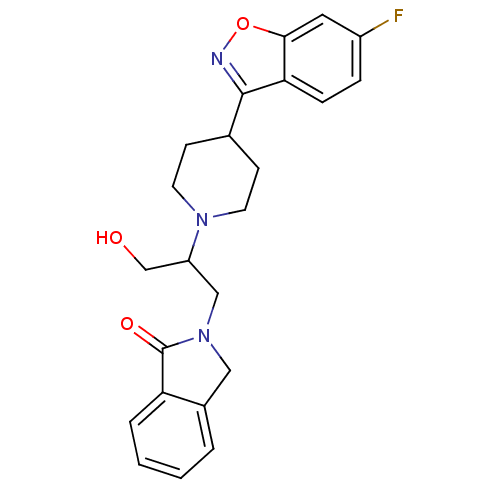
(Isoindoline, 23)Show SMILES OCC(CN1Cc2ccccc2C1=O)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H24FN3O3/c24-17-5-6-20-21(11-17)30-25-22(20)15-7-9-26(10-8-15)18(14-28)13-27-12-16-3-1-2-4-19(16)23(27)29/h1-6,11,15,18,28H,7-10,12-14H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
All rat alpha-1 adrenoceptor binding was performed by Hugo M. vargas, Karen M. Brooks and Lynn Laws-Ricker. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156457
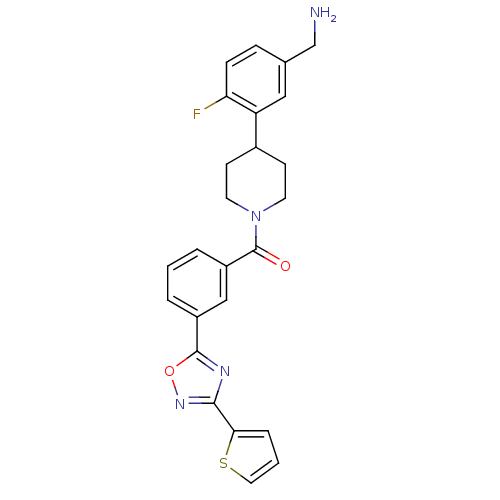
(CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cccc(c1)-c1nc(no1)-c1cccs1 Show InChI InChI=1S/C25H23FN4O2S/c26-21-7-6-16(15-27)13-20(21)17-8-10-30(11-9-17)25(31)19-4-1-3-18(14-19)24-28-23(29-32-24)22-5-2-12-33-22/h1-7,12-14,17H,8-11,15,27H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84448
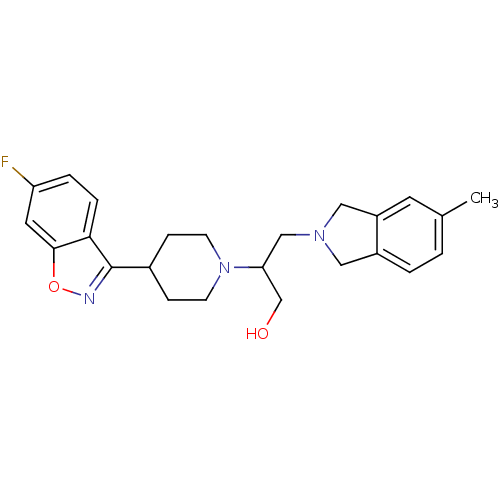
(Isoindoline, 15)Show SMILES Cc1ccc2CN(CC(CO)N3CCC(CC3)c3noc4cc(F)ccc34)Cc2c1 Show InChI InChI=1S/C24H28FN3O2/c1-16-2-3-18-12-27(13-19(18)10-16)14-21(15-29)28-8-6-17(7-9-28)24-22-5-4-20(25)11-23(22)30-26-24/h2-5,10-11,17,21,29H,6-9,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM84456

(Isoindoline, 23)Show SMILES OCC(CN1Cc2ccccc2C1=O)N1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H24FN3O3/c24-17-5-6-20-21(11-17)30-25-22(20)15-7-9-26(10-8-15)18(14-28)13-27-12-16-3-1-2-4-19(16)23(27)29/h1-6,11,15,18,28H,7-10,12-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
| Assay Description
Dopamine D4.2 and D2S receptor binding was performed at NPS Allexlix Corp. |
Chembiochem 3: 999-1009 (2002)
Article DOI: 10.1002/1439-7633(20021004)3:10
BindingDB Entry DOI: 10.7270/Q27D2SP5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50085406

((2R,3R)-3-[(Biphenyl-4-carbonyl)-amino]-2-(3-carba...)Show SMILES COC(=O)[C@H](Cc1cccc(c1)C(N)=N)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H27N3O3/c1-17(23(26(31)32-2)16-18-7-6-10-22(15-18)24(27)28)29-25(30)21-13-11-20(12-14-21)19-8-4-3-5-9-19/h3-15,17,23H,16H2,1-2H3,(H3,27,28)(H,29,30)/t17-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human Coagulation factor Xa |
Bioorg Med Chem Lett 12: 1667-70 (2002)
BindingDB Entry DOI: 10.7270/Q2GM86MR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data